Materials for microfluidic diagnostics
“In vitro diagnostic(s)” (IVD(s)) is a term for a broad industry encompassing everything from benchtop or larger bioanalytical instrumentation applications such as protein analysis and next generation sequencing to point-of-care devices. Getting the biology right for IVD development is already a challenge. The instrumentation, platform, and consumables development add layers of complexity. Increasingly, microfluidics as an approach for the IVD consumable or platform is a strategy to employed to overcome these challenges. Selecting the best materials for microfluidic diagnostics requires taking into account the complexities of the biological components, the microfluidic device processing tools available for manufacture of these devices, and exploiting these tools and materials to bring out the best in the diagnostic performance.
Microfluidics, or lab-on-a-chip technology, is a powerful tool that sits at the intersection of biotechnology, automation, and functional integration. By using photolithography and other CMOS fabrication techniques to make fluid flow cells, microfluidic devices can shuttle picoliter, volumes of fluids into functional regions, enabling powerful in vitro diagnostics (IVD) harnessing everything from digital biology, next generation sequencing, organs-on-a-chip, high-throughput cell-based assays, or high-density multifunctional chips for drug discovery and bioanalysis for shrinking sample and reagent volumes.
Materials selected for the IVD device often define the patterning and processing tools and techniques applied (rather than the other way around) and ultimately the manufacturing, performance, functionality, and the cost of the device. Typical IVD device materials are primarily glass, silicon, and polymers (plastics and photoresists). However, paper, 3D printed materials, hybrid devices, and other new materials are emerging [1].
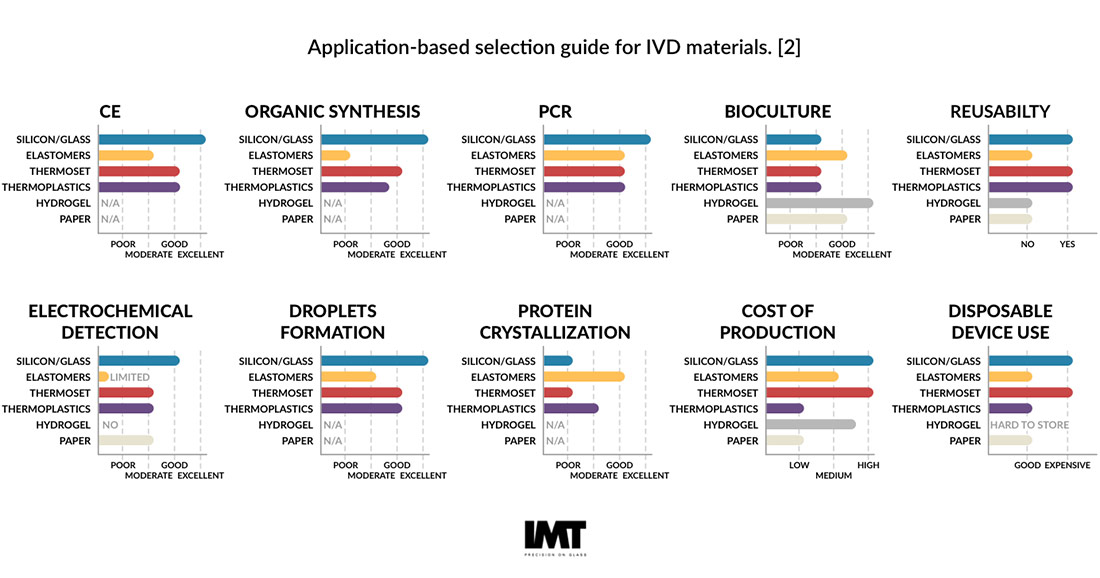
While the selection of the right materials for microfluidic diagnostics requires a good understanding of the function and design details of the in vitro diagnostic being designed, there are some general rules of thumb for when to use each material. Our infographic above shows the trade-offs based on function [2].
Glass, with favorable and well-understood physical, chemical, electrical, and thermal properties, is best for applications that require optical transparency, a low fluorescence background and a low channel surface roughness. Silicon is historically favored for the ability to achieve nano- to microscale high-aspect aspect ratio features and multifunctional layering. Polymers are primarily used to control manufacturing costs at the production scale [2].
Paper is emerging as a material for patterned lateral flow assays, but it does face some challenges in implementation, with the main drivers being ease of use and low cost. Additive methods, including the 3D printing of biocompatible and biological materials, have applications in the development of the so called “organ-on-a-chip” and “bioreactor-on-a-chip” technologies [1].
Thanks to advances in laser machining, photolithography, etching automation, wafer bonding, functionalization, room temperature UV-adhesive bonding and other process improvements, glass and glass-hybrid materials are often both the best-performing and most cost-effective consumable material for IVD devices.
Works Cited
[1] X. Hou, Y. S. Zhang, G. Trujillo-de Santiago, M. M. Alvarez, J. Ribas, S. J. Jonas, P. S. Weiss, A. M. Andrews, J. Aizenberg and A. Khademhosseini, "Interplay between materials and microfluidics," Nature Rev Mat, vol. 2, pp. 1-15, 2017.
[2] K. Ren, J. Zhou and H. Wu, "Materials for Microfluidic Chip Fabrication," Accounts of Chemical Research, vol. 46, no. 11, pp. 2396-2406, 2013.